Intravenous Filters
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices have helped optimize infusion therapy worldwide since 1960.
Problems
Air embolism: > one death per day alone in the US
The Problem
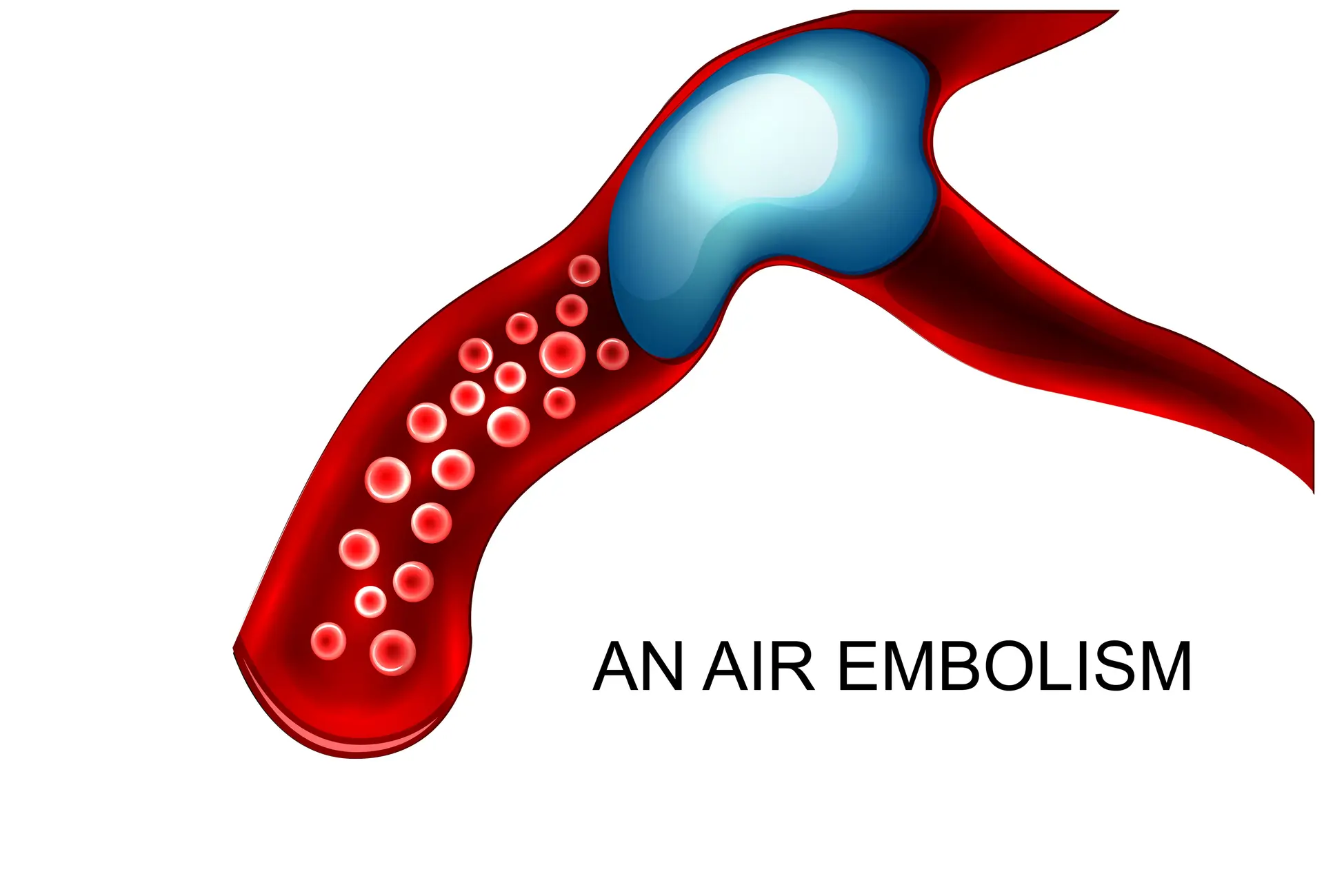
Air embolism is a preventable hospital-acquired condition that can result in serious harm, including death.1,2 Incidences of vascular air embolism costs the healthcare system, on average, $66,007 per incident.3 Even the most conservative estimates (5 million CVCs per year in US, air embolus 0.1% of CVCs, and 23% mortality) indicate 1,150 deaths per year from vascular air embolism in the US alone.4

Download Infographic 5 facts about vascular air embolism
The Solution
Air-eliminating filters are designed to remove inadvertent air which may be found in solutions for intravenous administration.3
The Proof
Our air-eliminating filters prevent inadvertent air from entering the vascular system of patients in need of intravenous therapies.
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).
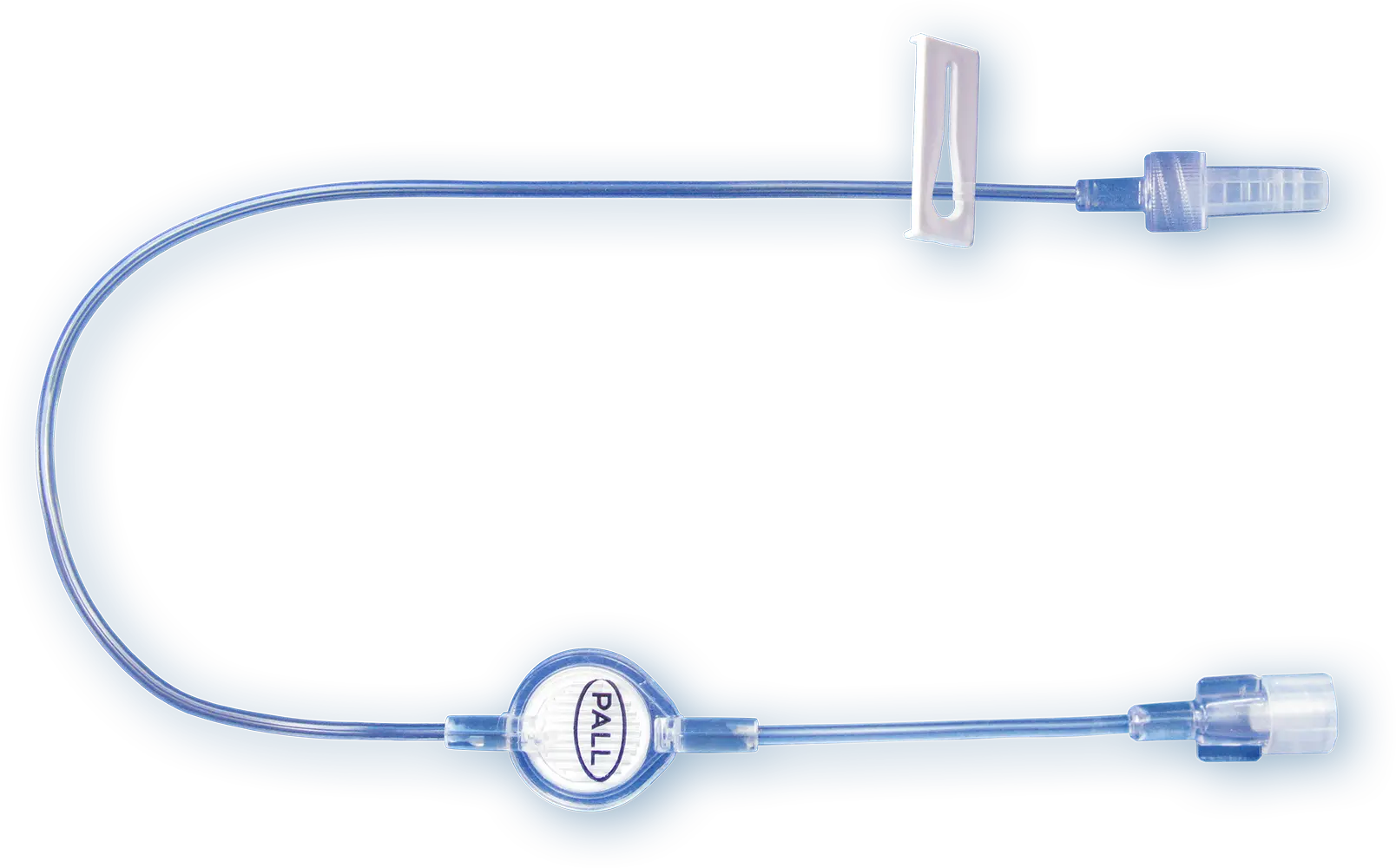
Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.
Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.
Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Feil M. (2012). Reducing Risk of Air Embolism Associated with Central Venous Access Devices. Pennsylvania Patient Safety Authority; 9 (2): 58-65
Center for Medicare and Medicaid Services. (2020). Publicly Reported DRA HAC Measures - Frequently Asked Questions from [accessed 02/23/2021]
Lee S. and Bulsara KR. (2020) Assessing the Efficacy of Commercially Available Filters in Removing Air Micro-Emboli in Intravenous Infusion Systems. J Extra Corpor Technol; 52(2):118-125
Patientsafe (2016) Central Lie Air Emboli: one death every day? [accessed on 01/02/2021]
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Organ failure rate in ICU patients: 1 in 7
The Problem

Roughly 15% patients develop organ failure in the ICU. The organ failures most commonly prevalent during the ICU stay are respiratory and renal failures1.

Download infographic Symptoms & manifestations in multiple organ failure
The Solution
Intensive care patients usually receive a multitude of IV infusions that, without IV in-line filters, can infuse up to one million particles per day.2-5 Infused particles may lead to a disturbance in the microcirculation and a compromised microvascular flow in vital organs may result in organ dysfunction of ICU patients.6-8 Studies mimicking real hospital scenarios have proven that our IV in-line filters can retain particles.9,10

Download publication snapshot Inline filters prevent capillary obstruction
The Proof
Studies suggest that IV in-line filters have a beneficial effect on the preservation of respiratory, renal, and hematologic organ function in critically ill patients.11-14
Download Infographic IV filters preventing organ dysfunction
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Pedersen PB. et al. (2017). Prevalence and prognosis of acutely ill patients with organ failure at arrival to hospital: protocol for a systematic review. Syst Rev; 6(1): 227
Perez M. et al. (2016). Particulate Matter in Injectable Drugs: Evaluation of Risks to Patients. Pharm. Technol. Hosp. Pharm.; 1(2): 91-103
Langille, S.E. (2013). Particulate Matter in Injectable Drug Products. PDA J Pharm Sci and Tech; 67: 186-200
Perez M. et al. (2015). In vitro analysis of overall particulate contamination exposure during multidrug IV therapy: impact of infusion sets. Pediatr Blood Cancer; 62(6): 1042-7
Benlabed M. et al. (2019). Clinical implications of intravenous drug incompatibilities in critically ill patients. Anaesth Crit Care Pain Med;38(2): 173-180
Lehr HA. et al. (2002). Particulate Matter Contamination of Intravenous Antibiotics Aggravates Loss of Functional Capillary Density in Postischemic Striated Muscle. Am J Respir Crit Care Med; 165: 514-520
Kirkpatrick CJ. et al. (2013). Non-Equivalence of Antibiotic Generic Drugs and Risk for Intensive Care Patients. Pharmaceut Reg Affairs; 2(1): 1-7
Schaefer S.C. et al. (2008). 0.2 µm in-line filters prevent capillary obstruction by particulate contaminants of generic antibiotic preparations in postischemic muscle. Chemother J; 17: 172-8
Perez M. et al. (2018). Effectiveness of in-Line Filters to Completely Remove Particulate Contamination During a Pediatric Multidrug Infusion Protocol. Sci Rep; 8 (7714): 1-8
Keck CM. (2016). How many nanoparticles enter a patient during infusion therapy? Journal of Vascular Access; 17(4): e8
Jack T. et al. (2012). In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Med; 38: 1008-1016
Boehne M. et al. (2013). In-line filtration minimizes organ dysfunction: New aspects from a prospective, randomized, controlled trial. BMC Pediatrics; 13 (21): 1-8
Sasse M. et al. (2015). In-line Filtration Decreases Systemic Inflammatory Response Syndrome, Renal and Hematologic Dysfunction in Pediatric Cardiac Intensive Care Patients. Pediatr Cardiol; 36: 1270-1278
Schmitt E. et al. (2019). In-line filtration of intravenous infusion may reduce organ dysfunction of adult critical patients. Critical Care; 23 (373): 1-11
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Costs of ICUs: up to 20% of a hospital's budget
The Problem

ICUs represent the largest clinical cost centres in hospitals, with expenses estimated to reach up to 20% of a hospital's budget1,2. The total cost per ICU patient highly depends on the length of the ICU stay3,4. Overall, mechanical ventilation is an important driver of ICU costs2.
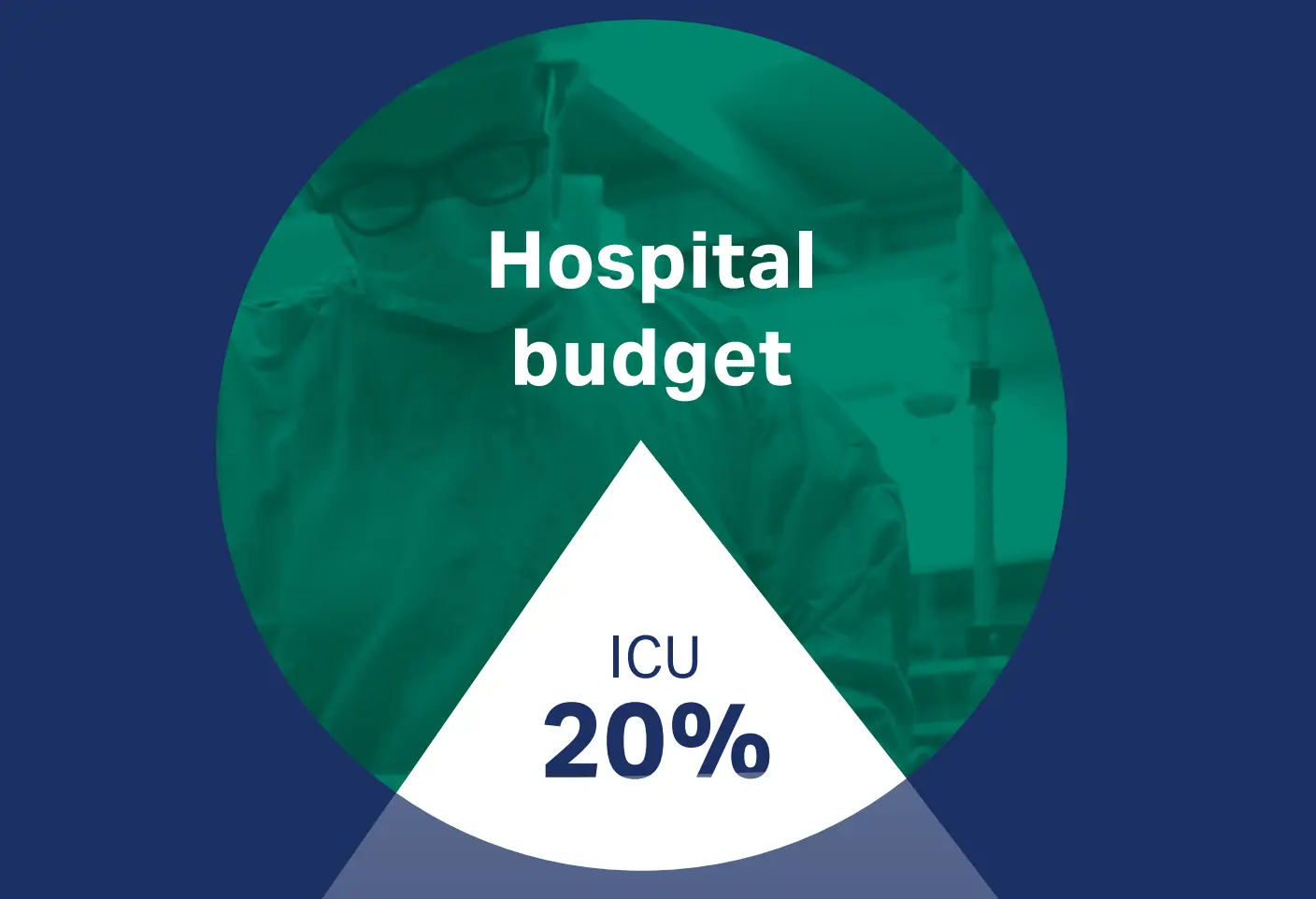
Download infographic ICU: the cost center of hospitals
The Solution
Our IV in-line filtration devices protects the patient against potential complications from infusion of inadvertent particulate and microbial contamination as well as associated endotoxins and entrained air 5-7,* and therefore mitigate additional risks, potentially leading to prolonged ICU stays.
*Please refer to product literature for performance summary
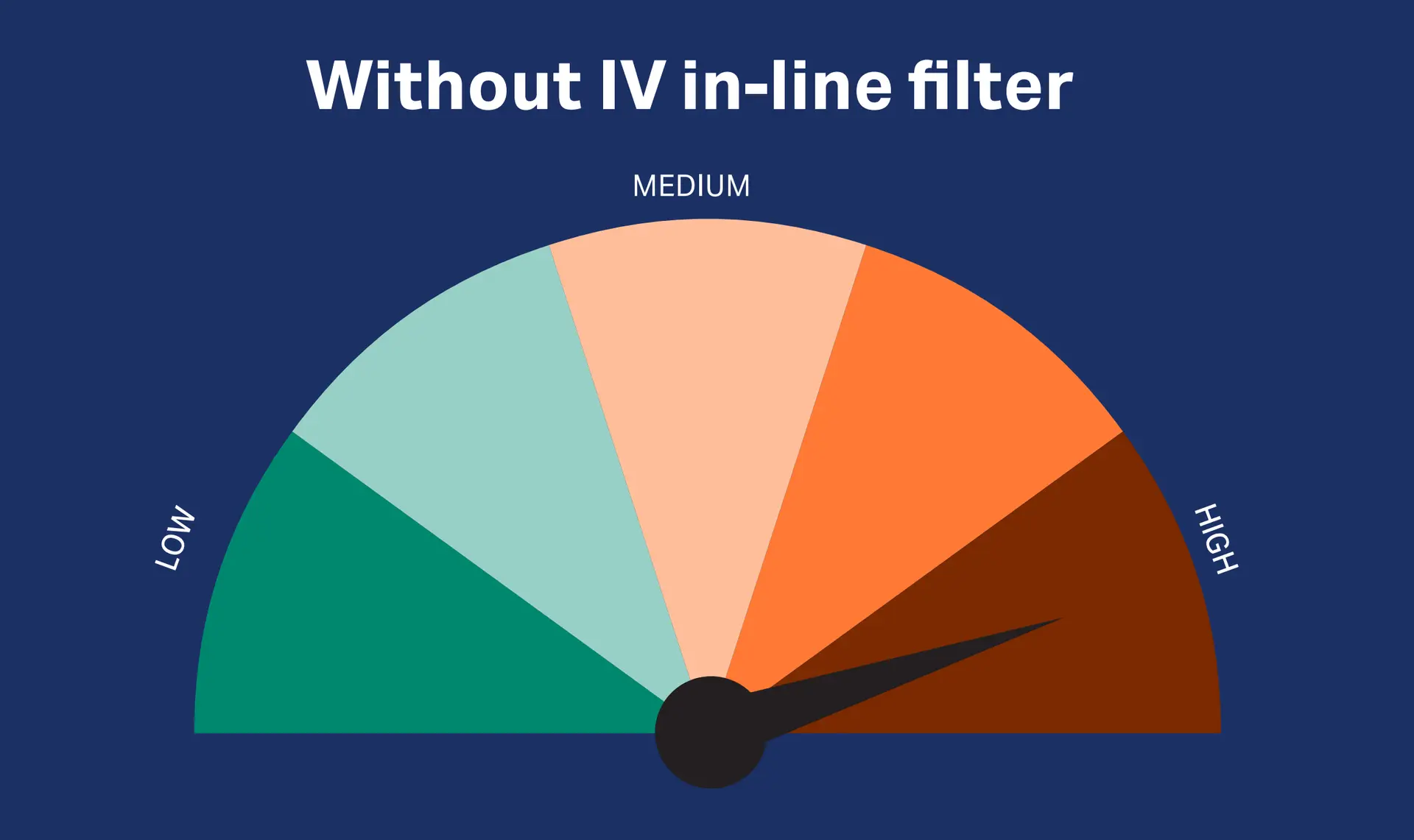
Download infographic Potential risks in IV therapy
The Proof
Studies demonstrated that ICU patients with IV in-line filters were able to leave the ICU significantly earlier than patients without IV in-line filters and IV in-line filters significantly reduce the duration of mechanical ventilation.8-11 An analysis evaluating the economic value of in-line filters revealed that an investment of 50K € in in-line filters led to a return of investment for the hospital of 1.6 million €.12

Download infographic Economic aspects of IV in-line filters
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Chalfin DB (1995). Cost-effectiveness analysis in health care. Hosp Cost Manag Account; 7:1-8
Kaier K, Heister T, Wolff J, Wolkewitz M. Mechanical ventilation and the daily cost of ICU care. BMC Health Serv Res. 2020 Mar 31;20(1):267
Rapoport J. et al. (1990). Explaining variability of cost using a severity-of-illness measure for ICU patients. Med Care; 28: 338-348
Higgins TL. et al. (2003). Early indicators of prolonged intensive care unit stay: impact of illness severity, physician staffing, and pre-intensive care unit length of stay. Crit Care Med; 31:45-51
Perez M. et al. (2018). Effectiveness of in-Line Filters to Completely Remove Particulate Contamination During a Pediatric Multidrug Infusion Protocol. Sci Rep; 8 (7714): 1 – 8
Ragunath S. & Spiers S. (2021). Evaluation of endotoxin retention efficiency of Pall ELD96 IV filters with 0.2 µm Posidyne® membrane over a 96-hour period; Pall Technical Report
Ragunath S. & Spiers S. (2021). Evaluation of endotoxin retention efficiency of Pall NEO96 IV filters with 0.2 µm Posidyne® membrane over a 96-hour period; Pall Technical Report
Jack T. et al. (2012). In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Med; 38: 1008-1016
Boehne M. et al. (2013). In-line filtration minimizes organ dysfunction: New aspects from a prospective, randomized, controlled trial. BMC Pediatrics; 13 (21): 1-8
Sasse M. et al. (2015). In-line Filtration Decreases Systemic Inflammatory Response Syndrome, Renal and Hematologic Dysfunction in Pediatric Cardiac Intensive Care Patients. Pediatr Cardiol; 36: 1270-1278
Schmitt E. et al. (2019). In-line filtration of intravenous infusion may reduce organ dysfunction of adult critical patients. Critical Care; 23 (373): 1-11
Unger-Hunt L. (2019). Reducing Risks and Generating Economic Benefits. Health Management; 19(4): 286-287
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Peripheral phlebitis: > 1 out of 10 ICU patients

The Problem
The most common and important peripheral intravascular catheters (PIVCs)-related complication is phlebitis. PIVC-related phlebitis occurred in 12.9% of ICU patients. Phlebitis causes pain, anxiety and therapy interruption. It can also potentially lead to serious complications (e.g., skin necrosis, infective endocarditis)1.
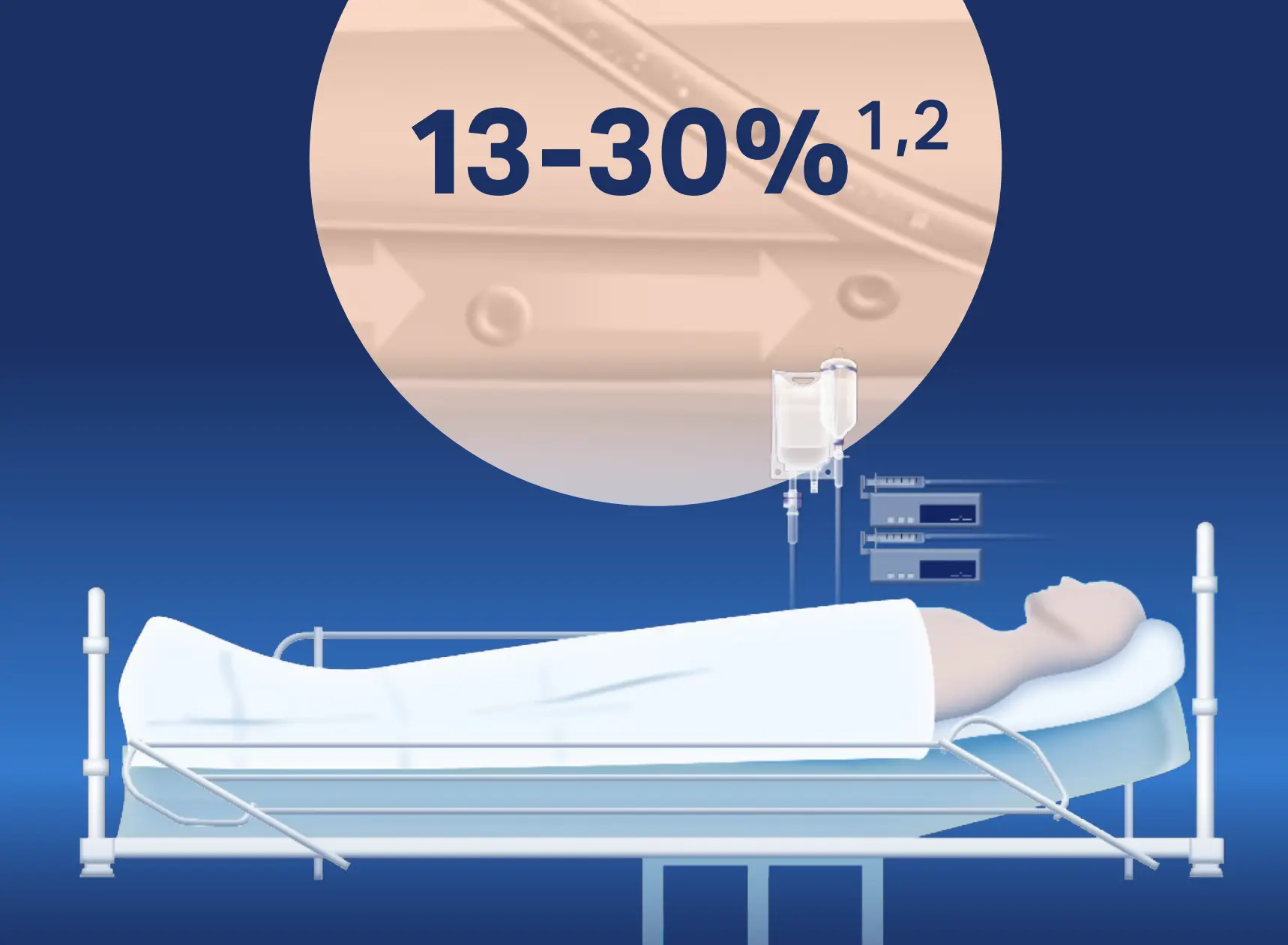
Download infographic: ICU patients suffer from peripheral phlebitis
The solution
The inadvertent intravenous infusion of particles has been identified as a risk factors for phlebitis.2-4 Studies mimicking real hospital scenarios have proven that our IV in-line filters can retain inadvertent particulate debris.5,6
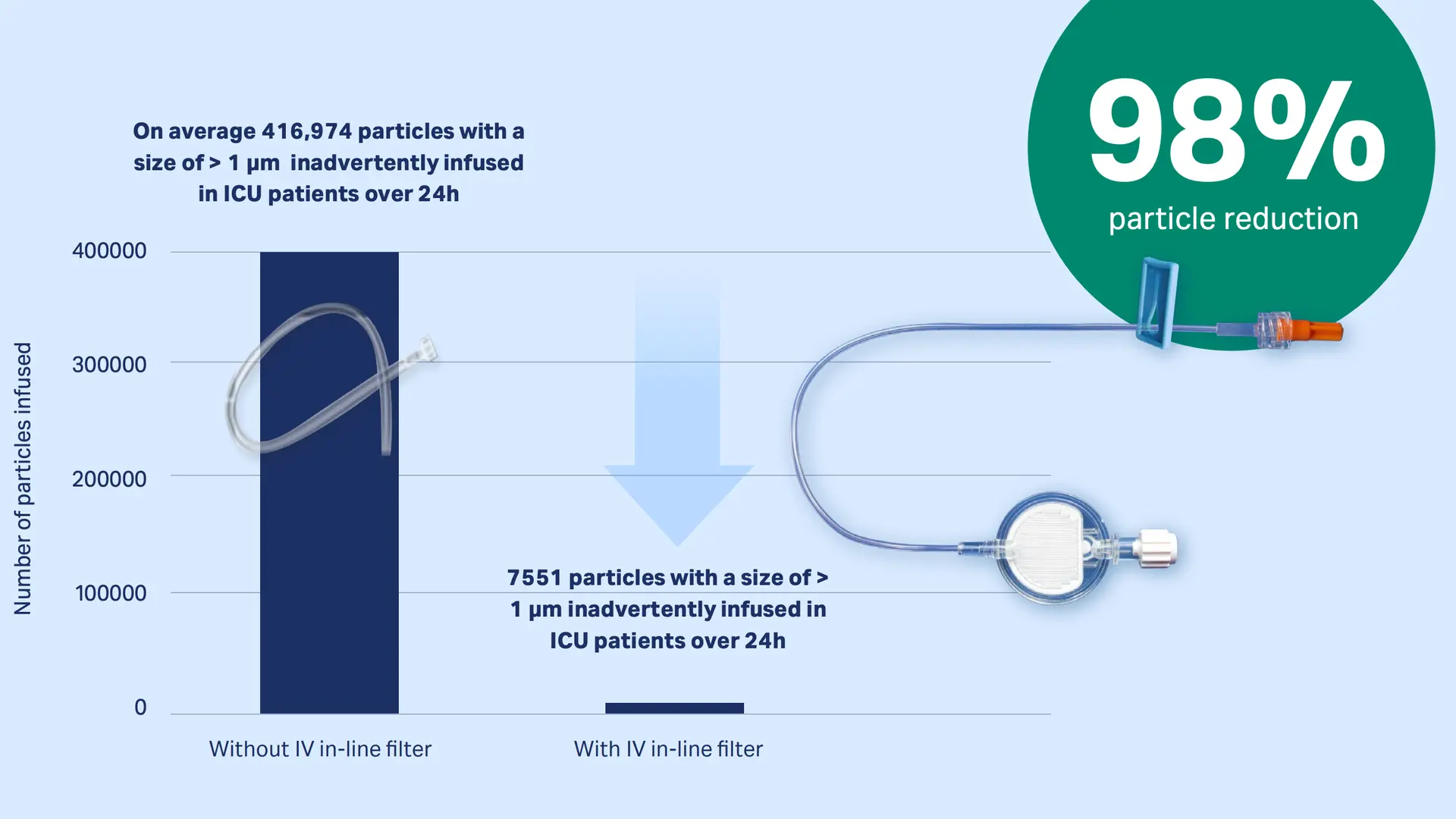
Download publication snapshot IV infusion may reduce organ dysfunction
The Proof
In a recent study with a cohort of 268 surgical patients, the incidence of phlebitis within 48 postoperative hours was 2.2% for patients in the in-line filter group versus 26.9% for those in the no-filter group.7
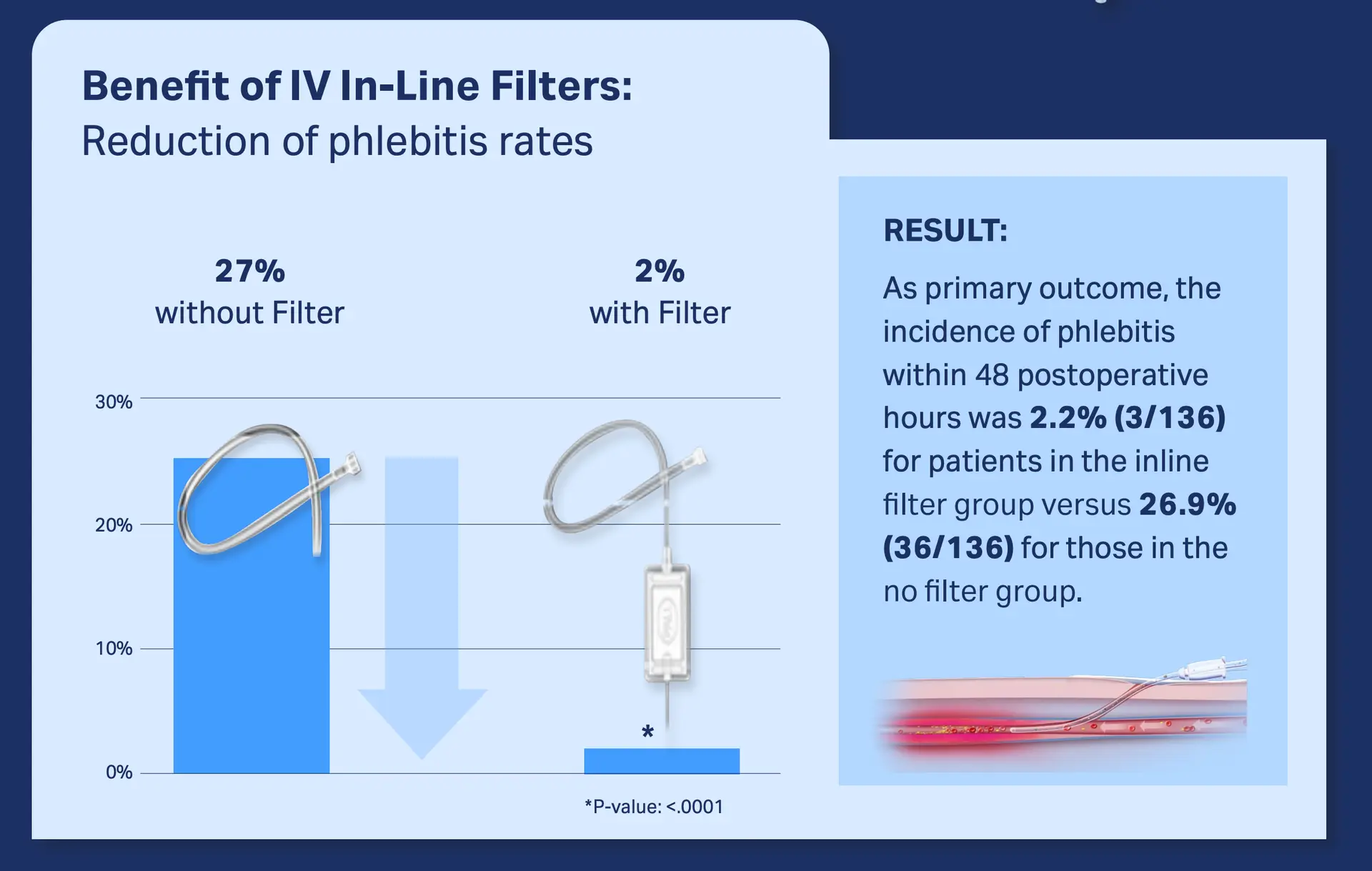
Download publication snapshot: In-line filtration reduces postoperative venous phlebitis
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Yasuda H. et al. (2021). AMOR-VENUS study group. Occurrence and incidence rate of peripheral intravascular catheter-related phlebitis and complications in critically ill patients: a prospective cohort study (AMOR-VENUS study). J Intensive Care; 9(1):3
Ball PA. (2001). Particulate contamination in parenteral nutrition solutions: still a cause for concern? Nutrition; 17:926–929
Falchuk KH. (1985). Microparticulate-induced phlebitis. Its prevention by in-line filtration. N Engl J Med; 312(2):78-82
Perez M. et al. (2018). Effectiveness of in-Line Filters to Completely Remove Particulate Contamination During a Pediatric Multidrug Infusion Protocol. Sci Rep; 8 (7714): 1-8
Keck CM. (2016). How many nanoparticles enter a patient during infusion therapy? Journal of Vascular Access; 17(4): e8
Villa G. et al. (2018). In-Line Filtration Reduces Postoperative Venous Peripheral Phlebitis Associated With Cannulation: A Randomized Clinical Trial. Anesth Analg; 127(6): 1367-1374
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Immunogenicity: up to 70% of patients
The Problem

Drug immunogenicity manifests in the generation of anti-drug antibodies (ADAs), and some mAbs show immunogenicity in up to 70% of patients1. Particles in drug products can pose potential immunogenic risks in patients2-4.
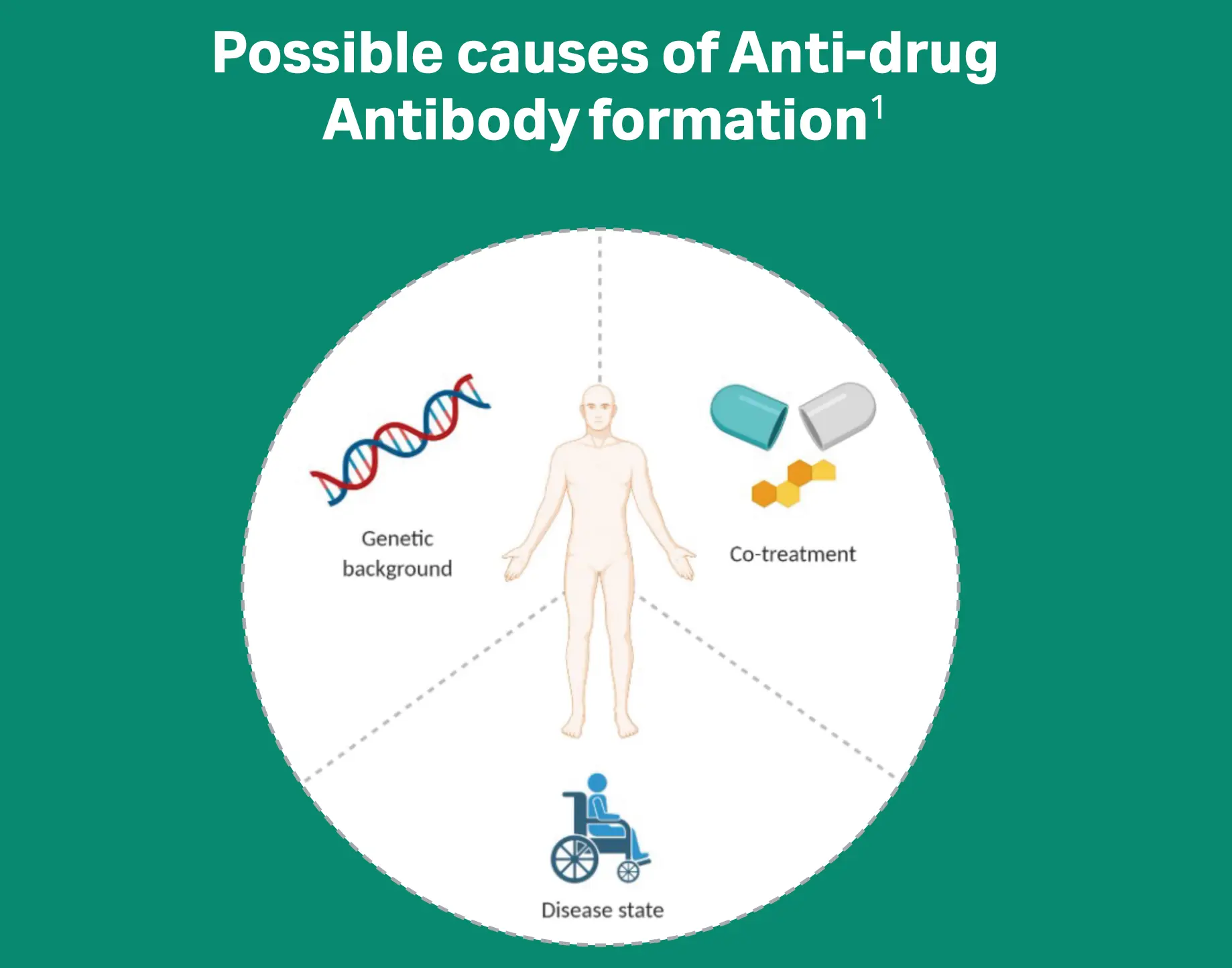
Download the infographic Immunogenicity of monoclonal antibodies
The Solution
Many monoclonal antibody (mAB) drug manufacturers recommend or require the use of filters in their instructions for use and the retention performance of inadvertent particles present in mAbs with our filters has been demonstrated.4
Read our blog Does your IV drug requires filtration?
The Proof
Our Technical Report “Binding of Monoclonal Antibodies to Pall Supor™ AEF” demonstrates that our Supor AEF filters (AEF1E and AEF1NTE) are low protein binding as often required by drug manufacturers.5

Download our Technical Report Binding of Monoclonal Antibodies
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Vaisman-Mentesh A. et al. (2020). The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front Immunol;11:1951.
Federal Register. 2014. Guidance for Industry on Immunogenicity Assessment for Therapeutic Protein Products; Availability. [online] Available at: [Accessed 11 November 2021].
Ratanji KD. et al. (2014). Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol; 11(2): 99-109
Werner BP and Winter G. (2018). Expanding Bedside Filtration-A Powerful Tool to Protect Patients From Protein Aggregates. J Pharm Sci; 107(11): 2775-2788
Saunders D & Stenning M. (2021). Binding of Monoclonal Antibodies to Pall Supor™ AEF Intravenous Filters; Pall Technical Report #210112.1IGL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Amiodarone phlebitis rate: as high as up to 85%
The Problem

Intravenous administration of amiodarone, the gold-standard treatment for arrhythmias, frequently leads to phlebitis1. Several studies reported extremely high phlebitis rates, ranging from 36% to 58% and as high as 85%1-6.

Download infographic: Phlebitis rates associated with Amiodarone
The Solution
Studies suggest that that the use of in-line filters and nursing guidelines significantly reduces phlebitis rates and phlebitis severity.1,5
Read our blog: Amiodarone and phlebitis - can filters help?
The Proof
We have carried out simulated testing with our ELD96 and AEF1 filters for Amiodarone.6
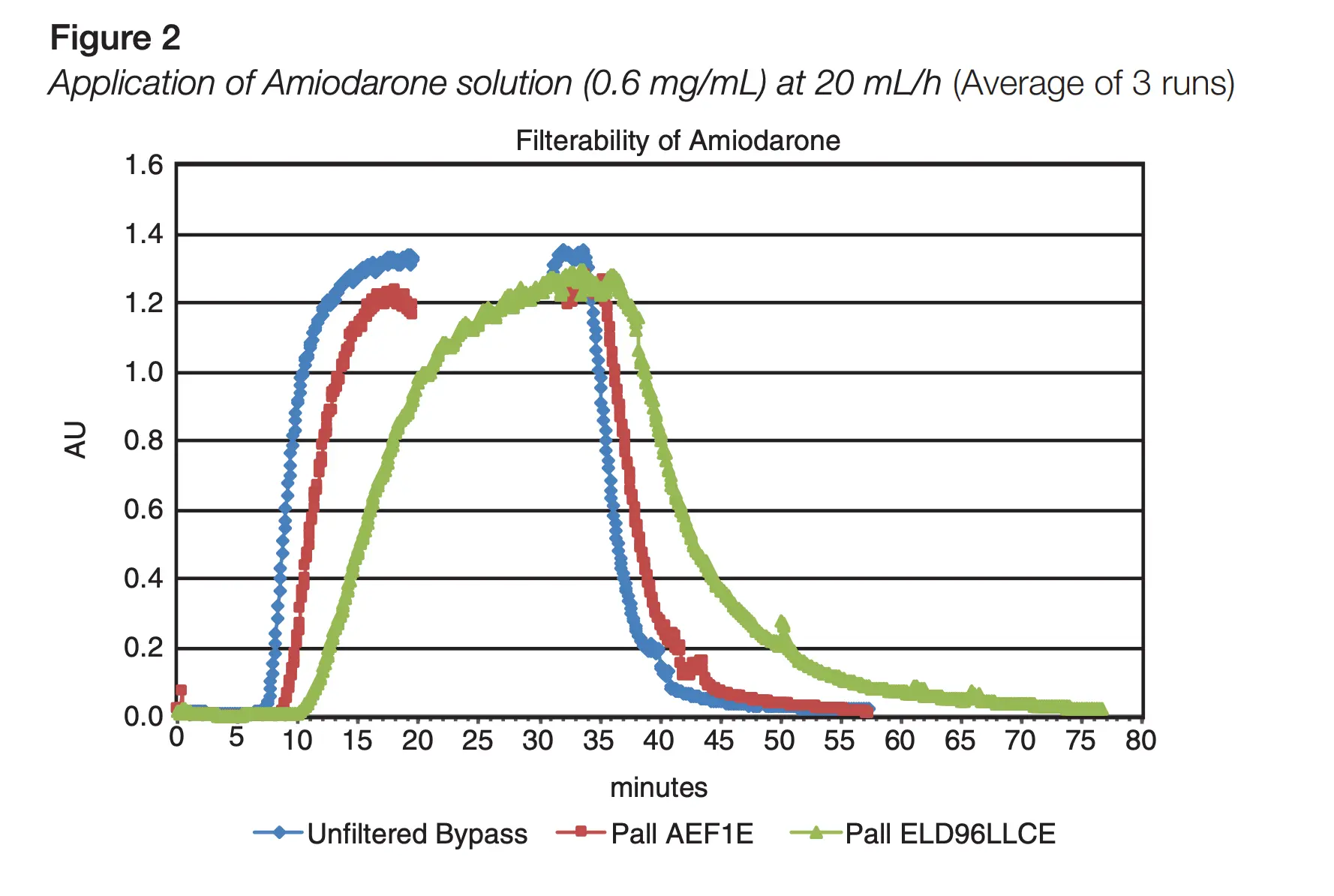
Download our Technical Report Filterability of Amiodarone Through Pall ELD Family and AEF1E Filters
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Oragano CA. et al. (2019). Phlebitis in Intravenous Amiodarone Administration: Incidence and Contributing Factors. Critical Care Nurse; 39 (1): e1–e12
Infusion Nurses Society (2007). Infusion nursing standards of practice. J Infus Nurs; 30(3)
Boyce BA and Yee BH. (2012). Incidence and severity of phlebitis in patients receiving peripherally infused amiodarone. Crit Care Nurse; 32(4):27-34
Martinho FS. and Rodrigues AB. (2008). Occurrence of phlebitis in patients on intravenous amiodarone. Einstein;6(4): 459-462
Norton L. et al. (2013). Phlebitis in amiodarone administration: incidence, contributing factors, and clinical implications. Am J Crit Care; 22(6):498-505
Bagheri-Nesami M, Shorofi SA, Hashemi-Karoie SZ, Khalilian A. The effects of sesame oil on the prevention of amiodarone-induced phlebitis. Iran J Nurs Midwifery Res. 2015;20(3):365-370.
Capewell A. (2018). Filterability of Amiodarone Through Pall ELD Family and AEF1E Filters; Pall Technical Report #180115.1IGL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Do you know the guidelines regarding the use of IV filters?
The Problem

In light of laboratory and clinical trial results demonstrating the benefits of IV in-line filters on intensive care patients, national and global acting medical societies advocate for the use of IV in-line filters. A publication in 2018 demonstrates the need for further ongoing dissemination and education on standardized safe practices, such as the use of IV in-line filter.1

Download infographic: Use of IV in-line filters and PN solutions
The Solution
Following guidelines recommend the use of IV-in line filters: American Society for Parenteral and Enteral Nutrition (ASPEN)2, Infusion Nursing Society (INS)3, European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN), European Society for Parenteral and Enteral Nutrition (ESPEN), European Society for Paediatric Research (ESPR) and Chinese Society for Parenteral and Enteral Nutrition (CSPEN)4, Irish Society for Clinical Nutrition & Metabolism (IRSPEN)5, French Health Authority (HAS)6, British Pharmaceutical Nutrition Group Working Party (BPNG)7, Commission for Hospital Hygiene and Infection Prevention (KRINKO)8

Download infographic of Guidelines recommending IV in-line Filters
The Proof
ASPEN: “Based on best available evidence and guidance from scientific and regulatory agencies, ASPEN recommends using a 1.2 micron in-line filter for administration of TNAs ( Total nutrient admixtures), dextrose-amino acid admixtures and ILE (Lipid Injectable Emulsion).”2
INS: "In-line filters were initially developed for infection control purposes, but their role in protecting patients from the harmful effects of particulate matter has emerged as their primary purpose in infusion therapy.”3
ESPGHAN, ESPEN, ESPR, CSPEN: “PN solutions may be administered through a terminal filter: lipid emulsions (or all-in-one mixes) can be passed through a membrane pore size of 1.2-1.5 µm; aqueous solutions can be passed through a 0.22 µm filter (GPP, strong recommendation for, strong consensus.”4
IRSPEN: “It is recommended that all PN solutions are administered via an infusion set containing a terminal filter.”5 HAS: “Use antibacterial (0.22 µm) and particulate (1.2 µm) in-line filters. Antibacterial filters cannot be used with lipids.”6
BPNG: “The 1.2-µm filters should be used for the administration of lipid-containing solutions including AIO admixtures and changed every 24 h. “The 0.2-µm endotoxin-retaining filters should be used for the administration of non–lipid-containing solutions and can be changed every 96 h.”7
KRINKO: "In intensive care patients particle filters should be used in the infusion system for patients."8
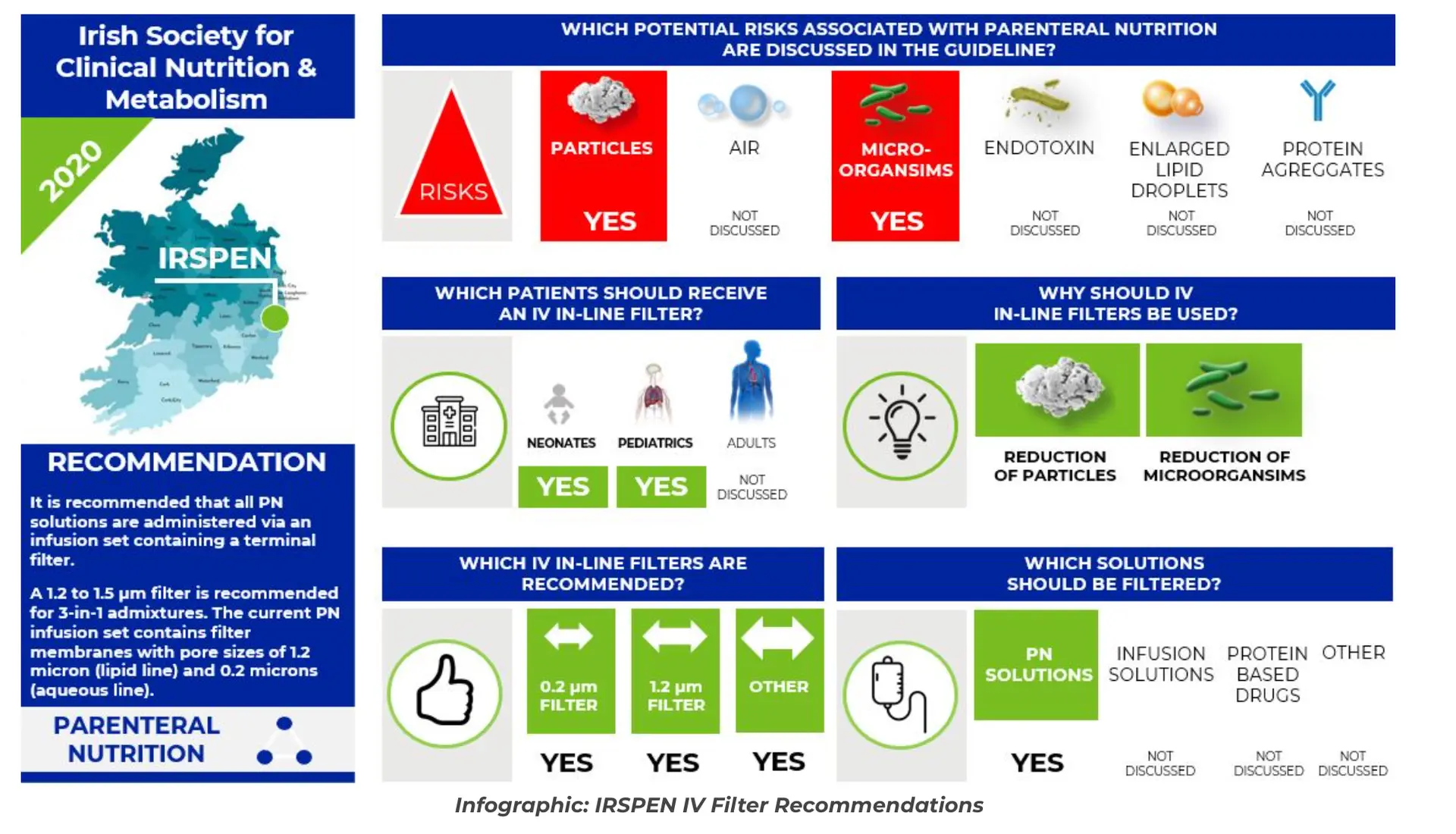
Read blog on 8 guidelines around the world recommending IV filters
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Christensen ML. (2017). Lipid Injectable Emulsion Survey With Gap Analysis. Nutr Clin Pract; 32(5): 694-702
Worthington P. et al. (2021). Update on the Use of Filters for Parenteral Nutrition: An ASPEN Position Paper. Nutr Clin Pract; 36(1): 29-39
Gorski L.A. et al. (2021). Infusion therapy standards of practice. J Infus Nurs; 44(suppl 1): S1-S224
Puntis JWL. et al. (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Organisational aspects. Clinical Nutrition; 37: 2392-2400
Irish Society for Clinical Nutrition & Metabolism. 2020. Guideline on the Use of Parenteral Nutrition in Neonatal and Paediatric Units. Accessed from https://www.hse.ie/eng/about/who/cspd/ncps/paediatrics-neonatology/resources/guideline-on-the-use-of-parenteral-nutrition-in-neonatal-and-paediatric-units.pdf
Haute Autorité de Santé (2018). Recommandation de bonne pratique. Nutrition parentérale en néonatologie. French.
Bethune K. et al. (2001). Use of Filters During the Preparation and Administration of Parenteral Nutrition: Position Paper and Guidelines Prepared by a British Pharmaceutical Nutrition Group Working Party. Nutrition;17(5): 403-8
KRINKO (2017). Prävention von Infektionen, die von Gefäßkathetern ausgehen. Bundesgesundheitsbl; 60:171–206 (German)
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Endotoxin production in IV solutions: risk to patients within 24 hours
The Problem

Previous studies have shown that gram-negative bacteria can multiply and will shed endotoxin in intravenous solutions within 24 hours.1,2 Hospital cases of endotoxin in intravenous solutions leading to adverse reactions or even death have been investigated.3-8
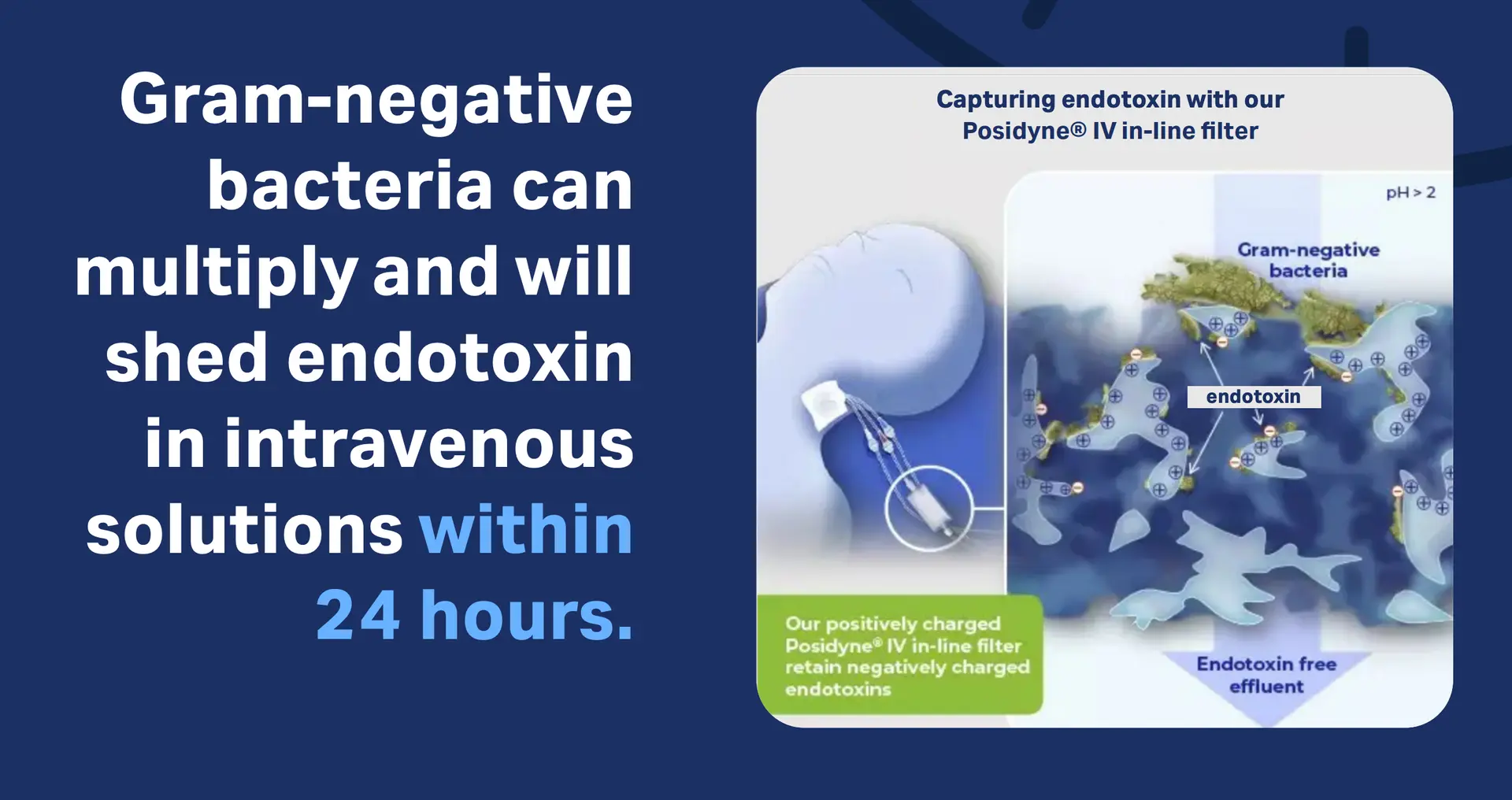
Download the infographic The risk - endotoxins of gram-negative bacteria
The Solution
Several publications have shown that our IV in-line filters with positively charged membranes retain endotoxin in both clinical and laboratory settings.9-15

Download infographic Capturing endotoxin
The Proof
We manufacture positively charged, membrane-based intravenous filter products with a claim for the removal of inadvertent microbial contaminants and associated endotoxins up to 96 hours.* Our technical reports demonstrate that our ELD96 and NEO96 IV in-line filters (aged and unaged) retain endotoxins.16,17
*Please refer to product literature for performance summary
Download our Technical Report Endotoxin retention effiency of Pall NEO96 IV filters
Download our Technical Report Endotoxin retention effiency of Pall ELD96 IV filters
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Holmes C.J. et al. (1980). Potential Hazards Associated with Microbial Contamination of In-Line Filters During Intravenous Therapy. Journal of Clinical Microbiology; 12 (6): 725-731
Trautmann M. et al. (1997). Bacterial colonization and endotoxin contamination of intravenous infusion fluids. J Hosp Infect; 37(3): 225-36
Garrett D.O. et al. (2002). An Outbreak of Neonatal Deaths in Brazil Associated with Contaminated Intravenous Fluids. The Journal of Infectious Diseases; 186 (1): 81–86
Daufenbach, L. (2006). Pyrogenic Reactions and Hemorrhage Associated With Intrinsic Exposure to Endotoxin-Contaminated Intravenous Solutions. Infection Control & Hospital Epidemiology; 27 (7): 735-741
Schroeder J. et al. (2015). Practically Saline. Journal of Investigative Medicine High Impact Case Reports; 1-4
CDC (1998). Endotoxin-Like Reactions Associated with Intravenous Gentamicin -- California, 1998. Retrieved from: https://www.cdc.gov/mmwr/preview/mmwrhtml/00055322.html
Johnstone T. et al. (2018). Seven cases of probable endotoxin poisoning related to contaminated glutathione infusions. Epidemiol Infect. 2018;146(7):931-934
Patel AS. et al. (2006) Outbreak of systemic inflammatory response syndrome linked to a compounding pharmacy – Virginia, 2005 In. 55th Annual Epidemic Intelligence Service
Baumgartner TG et al. (1986). Bacterial endotoxin retention by inline intravenous filters. Am. J. Hosp. Pharm; 43:681-684
Horibe K. et al. (1990). Evaluation of the endotoxin retention capabilities of inline intravenous filters. JPEN J. Parenter. Enteral. Nutr; 14: 56-59
Richards C. and Grassby P. F. (1994). A comparison of the endotoxin-retentive abilities of two ‘96-h’ in-line intravenous filters. J. Clin. Pharm. Ther; 19 (3): 199-202
Richards C. and Thomas P. (1990). Use of endotoxin retentive intravenous filters with paediatric total parenteral nutrition solutions. J Clin Pharm Ther;15(1): 53-8
Vanhaecke E. et al. (1989). Endotoxin removal by end-line filters. J Clin Microbiol; 27(12):2710-2
Barnett M.L. and Cosslett A.G. (1996). Endotoxin Retention Capabilities of Positively Charged Nylon and Positively Charged Polysulphone Membrane Intravenous Filters. Pharmacy and Pharmacology Communications; 2 (7): 319-320
Ortolano G. et al. (2009). Bacterial Lipopolysaccharide Retention by a Positively Charged Filter. Applied and Environmental Microbiology 75 (4): 1219
Ragunath S. and Spiers S. (2021). Evaluation of endotoxin retention efficiency of Pall NEO96 IV filters with 0.2 µm Posidyne® membrane over a 96-hour period. Pall Technical Report #210920.4GL
Ragunath S. and Spiers S. (2021). Evaluation of endotoxin retention efficiency of Pall ELD96 IV filters with 0.2 µm Posidyne® membrane over a 96-hour period. Pall Technical Report #210920.3GL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Particles: The invisible risk to ICU patients
The Problem
Intensive care patients usually receive a multitude of IV infusions that, without IV in-line filters, can infuse up to one million particles per day1-4. Infused particles may lead to a disturbance in the microcirculation and a compromised microvascular flow in vital organs may result in organ dysfunction of ICU patients 5-7.
Read blog and watch video Particles in an Infusion Line Entering a Patient’s Bloodstream
The Solution
IV in-line filters can be used as a strategy to reduce the numbers of particles inadvertently infused into ICU patients8-11.
The Proof
Studies mimicking real hospital scenarios have proven that our IV in-line filters can retain up to 99% of infused particles (dependent on the particle sizes)12,13
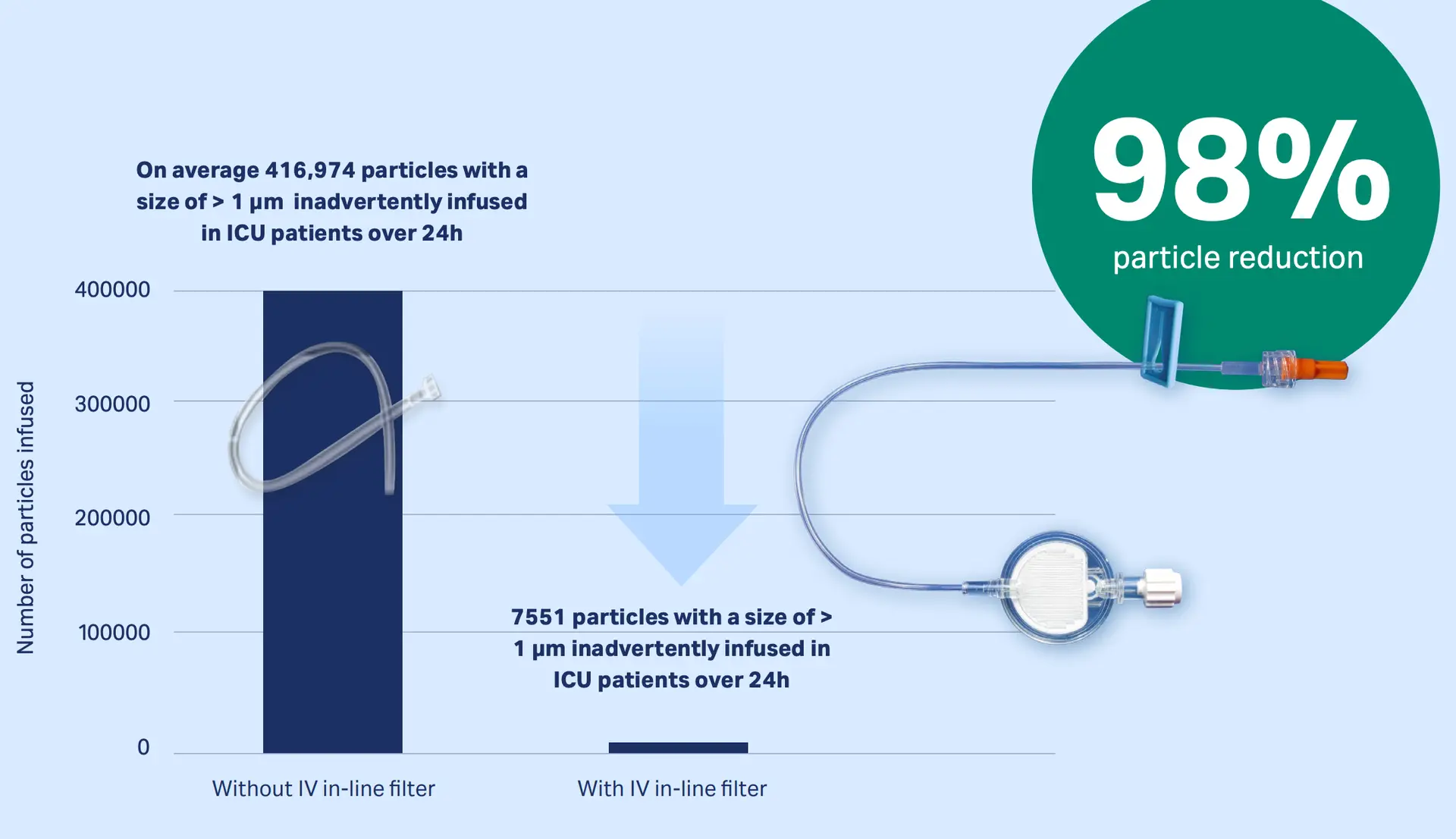
Download publication snapshot Retention of particles with IV in-line filters: 98%
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Perez M. et al. (2016). Particulate Matter in Injectable Drugs: Evaluation of Risks to Patients. Pharm. Technol. Hosp. Pharm.; 1(2): 91-103
Langille, S.E. (2013). Particulate Matter in Injectable Drug Products. PDA J Pharm Sci and Tech; 67: 186-200
Perez M. et al. (2015). In vitro analysis of overall particulate contamination exposure during multidrug IV therapy: impact of infusion sets. Pediatr Blood Cancer; 62(6): 1042-7
Benlabed M. et al. (2019). Clinical implications of intravenous drug incompatibilities in critically ill patients. Anaesth Crit Care Pain Med;38(2): 173-180.
Lehr HA. et al. (2002). Particulate Matter Contamination of Intravenous Antibiotics Aggravates Loss of Functional Capillary Density in Postischemic Striated Muscle. Am J Respir Crit Care Med; 165: 514-520
Kirkpatrick CJ. et al. (2013). Non-Equivalence of Antibiotic Generic Drugs and Risk for Intensive Care Patients. Pharmaceut Reg Affairs; 2(1): 1-7
Schaefer S.C. et al. (2008). 0.2 µm in-line filters prevent capillary obstruction by particulate contaminants of generic antibiotic preparations in postischemic muscle. Chemother J; 17: 172-8
Jack T. et al. (2012). In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Med; 38: 1008-1016
Boehne M. et al. (2013). In-line filtration minimizes organ dysfunction: New aspects from a prospective, randomized, controlled trial. BMC Pediatrics; 13 (21): 1-8
Sasse M. et al. (2015). In-line Filtration Decreases Systemic Inflammatory Response Syndrome, Renal and Hematologic Dysfunction in Pediatric Cardiac Intensive Care Patients. Pediatr Cardiol; 36: 1270-1278
Schmitt E. et al. (2019). In-line filtration of intravenous infusion may reduce organ dysfunction of adult critical patients. Critical Care; 23 (373): 1-11
Perez M. et al. (2018). Effectiveness of in-Line Filters to Completely Remove Particulate Contamination During a Pediatric Multidrug Infusion Protocol. Sci Rep; 8 (7714): 1-8
Keck CM. (2016). How many nanoparticles enter a patient during infusion therapy? Journal of Vascular Access; 17(4): e8
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Enlarged lipid droplets: safety concern for patients
The Problem
Overall, stabilization of lipid containing parenteral nutrition solutions is critical, since destabilization can cause the lipid globules to coalesce, thus causing the particle size to exceed 5µm. These particles will accumulate in pulmonary capillaries, which are between 4 and 9µm in diameter1.
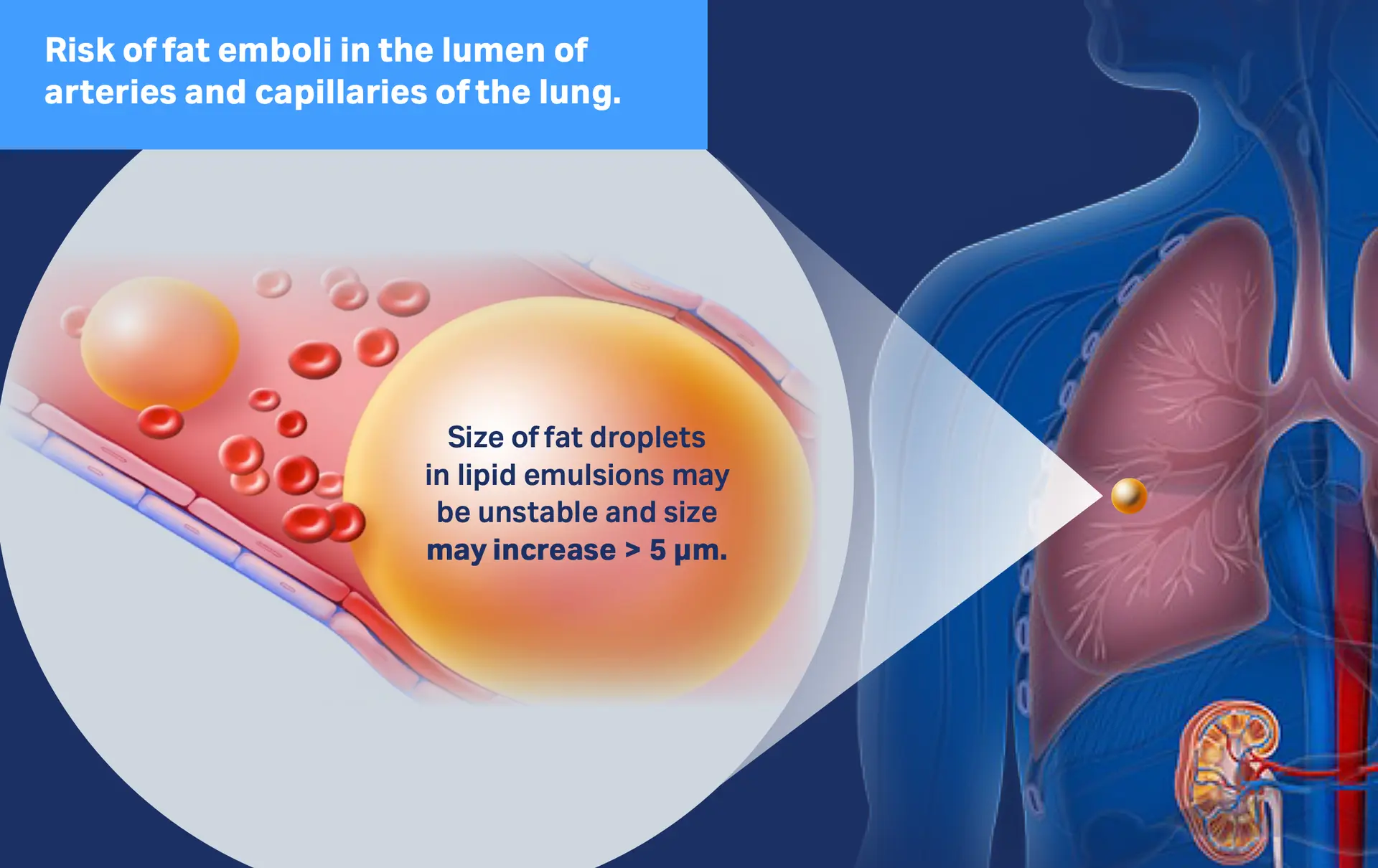
Download infographic Safety concern - enlarged lipid droplets
The Solution
1.2µm in-line filter retain large fat globules and serve a protective measurement2.

The Proof
Studies with our TNA 1.2 µm filter have demonstrated that total exposure to unstable and very large lipid droplets was significantly reduced, suggesting that our in-line TNA IV in-line filter should be a standard part of nutrition therapy.2,3
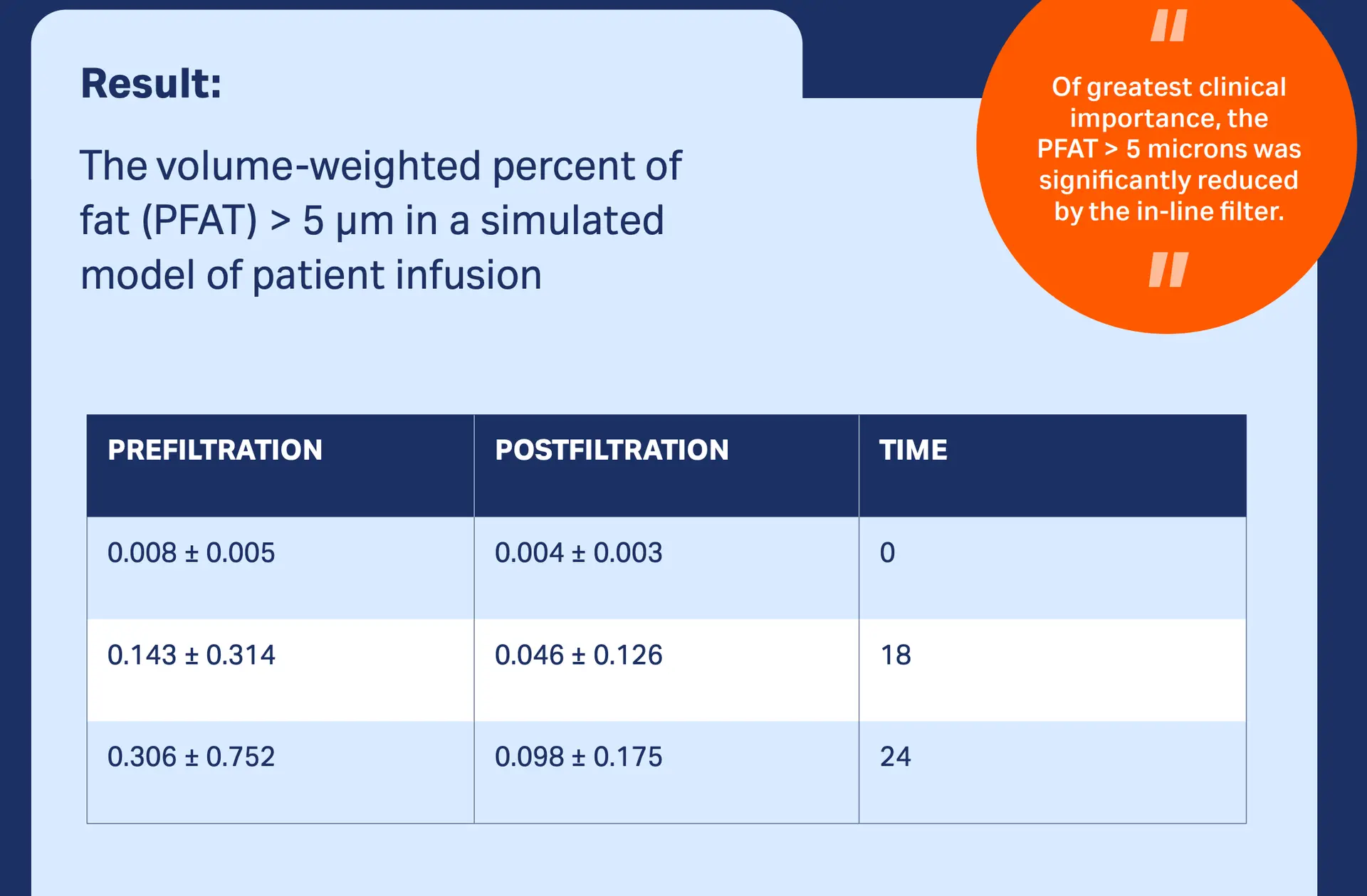
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Klang MG. (2015). PFAT5 and the Evolution of Lipid Admixture Stability. JPEN J Parenter Enteral Nutr; 39 (1 Suppl): 67S-71S
Bae JY et al. (2018) Sudden Deaths of Neonates Receiving Intravenous Infusion of Lipid Emulsion Contaminated with Citrobacter freundii. J Korean Med Sci; 33(10): e97
Driscoll DF. et al. (1996). Effects of in-line filtration on lipid particle size distribution in total nutrient admixtures. JPEN J Parenter Enteral Nutr; 20(4): 296-301
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Risk of intraluminal bacterial transfer and biofilm formation
The Problem
26% of central venous catheter-related blood stream infections appear to have had an intraluminal origin1.
Download infographic CVC-related bloodstream infections: intra- vs. extraluminal
The Solution
Studies concluded that the use of our 0.2 µm IV in-line filter significantly reduced the risk of bacterial transfer and biofilm formation within the catheter.2,3

The Proof
Our 0.2 µm in-line filters will remove inadvertent microbial contaminants where positively charged their associated endotoxins for up to 96 hours.* Our technical report demonstrates that our NEO96 & AEF1 filters acts as a barrier to any inadvertent microbial contaminants.4,5
Download the Technical Report Evaluation of bacterial retention efficiency of Pall NEO96 IV filters
*Please refer to product literature for performance summary
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
References
Safdar N. and Maki DG. (2004). The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med; 30(1): 62-7
Ryder M. (2016). Advanced IV filtration technology: Prevention of bacterial transfer and intraluminal biofilm formation. Journal of the Association for Vascular Access; 21(4): 247
Van Lingen R.A. et al. (2004). The use of in-line intravenous filters in sick newborn infants. Acta Paediatr; 93 (5): 658-62
Ragunath S. and Spiers S. (2021). Evaluation of bacterial retention efficiency of Pall NEO96 IV filters with 0.2 µm Posidyne® membrane over a 96-hour period. Pall Technical Report: #210920.1GL
Spiers S. (2022). Evaluation of bacterial retention efficiency of Pall Supor™ AEF IV Filters with 0.2 µm membrane over a 24 hour period. Pall Technical Report: #221006.1IG
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Implementing IV filter at the patient’s bedside
The Problem

Clinicians and pharmacists are concerned with selecting the appropriate filter for specific applications, such as drug preparation and intravenous (IV) in-line administration. Further, it is critical to know whether the IV filter can successfully administer the volume needed, at the appropriate flow rate, and for the required time.
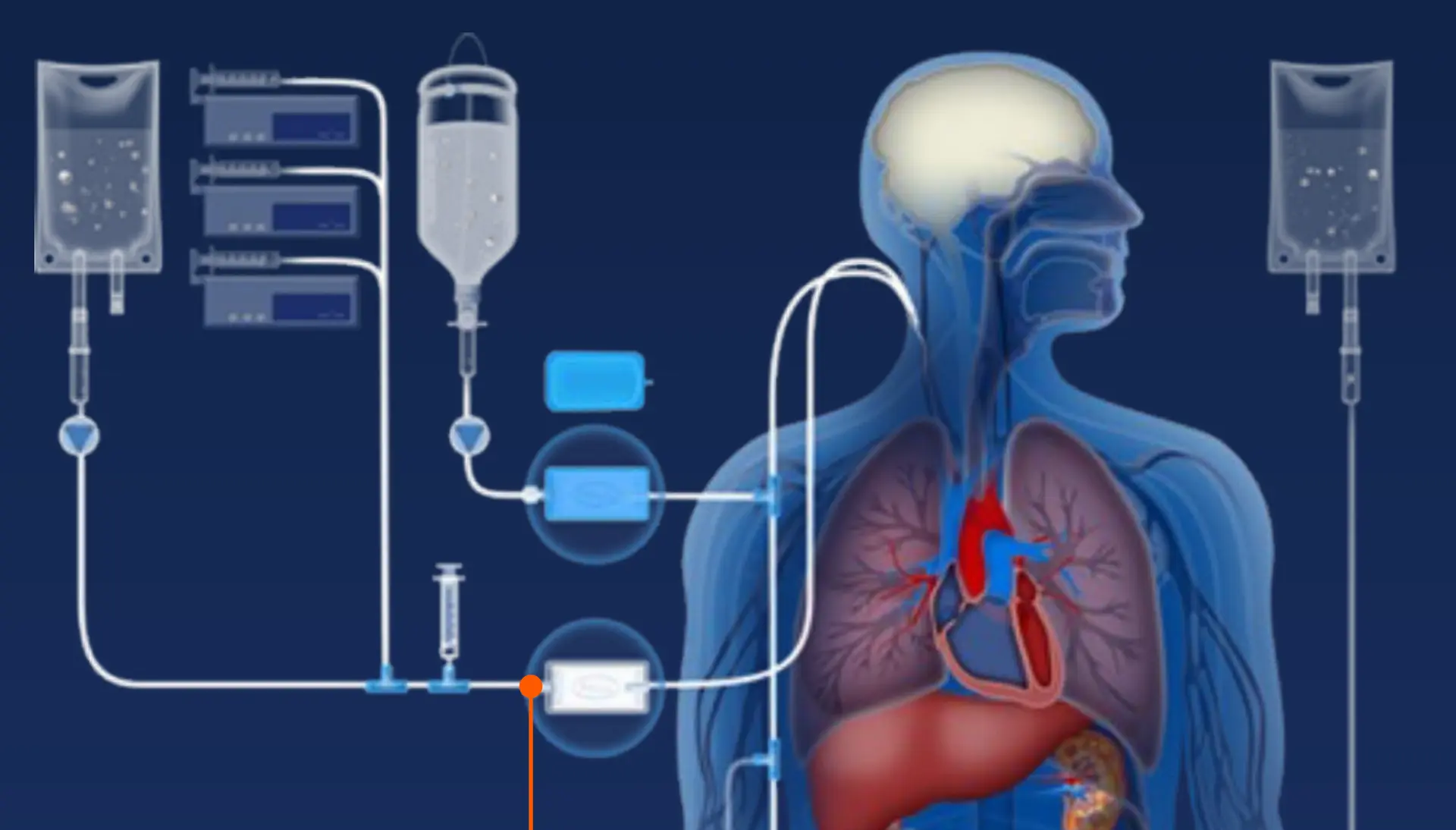
Download Infographic Intravenous therapies are complex
The Solution
Meet our Clinical Specialist Team. This team can support and train both new and existing users. Our Clinical Specialist Team offers hands-on care, before, during and after the installation of our IV filters.
Download Infograophic Our Clinical Specialist Team offers hands-on care
The Proof
Clinical Specialist Teams have been involved in implementing infusion regimes at the patient’s bedside all over the world.
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
IV Filter Performance
The Problem
Clinicians and pharmacists are concerned with selecting the appropriate filter for specific applications, such as drug preparation and intravenous (IV) in-line administration. Further, it is critical to know whether the IV filter can successfully administer the volume needed, at the appropriate flow rate, and for the required time.

Download infographic Critical to know
The Solution
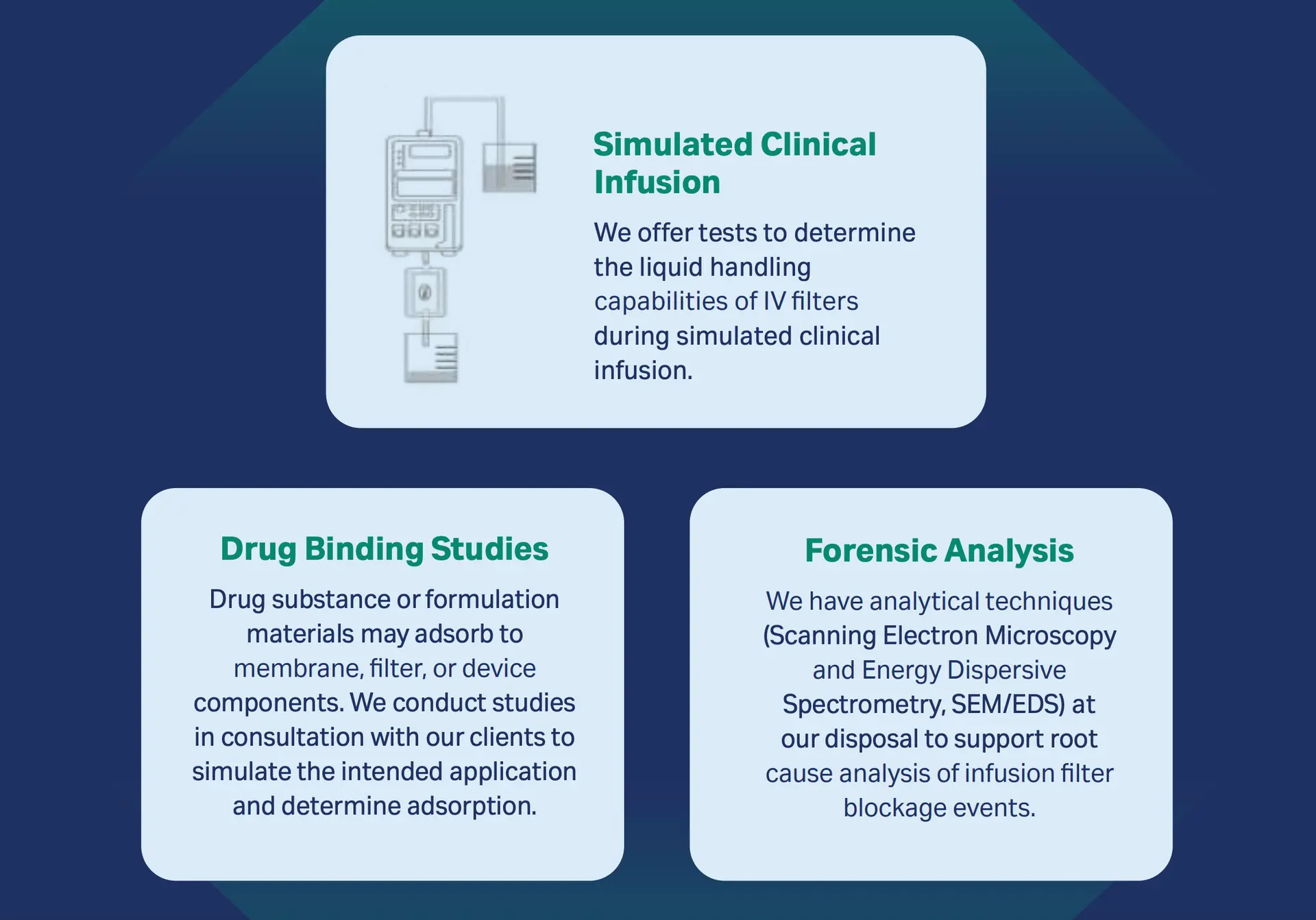
Meet our Scientific Laboratory Services (SLS) team. Our SLS team offers solutions for clinicians and pharmacists.
We offer the following:
Simulated Clinical Infusion
We offer tests to determine the liquid handling capabilities of IV filters during simulated clinical infusion.
Drug Binding Studies
Drug substance or formulation materials may adsorb to membrane, filter, or device components. We conduct studies in consultation with our clients to simulate the intended application and determine adsorption.
Forensic Analysis
We have analytical techniques (Scanning Electron Microscopy and Energy Dispersive Spectrometry, SEM/EDS) at our disposal to support root cause analysis of infusion filter blockage events.
Download infographic Take advantage of support
The Proof
We have a global network of scientists that for over 50 years have been dedicated to the provision of high quality applications support for our customers. With extensive experience in life science disciplines such as biochemistry, chemistry, microbiology and molecular biology, our applications experts have a proven track record in ensuring that our products are suitable for your chosen application.

Download infographic Over 50 Years experience
Our Products
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices and our customer and clinical body partnerships have helped optimize infusion therapy worldwide. Our in-line IV filters are developed for infusion managers, ICU physicians and nurses, pharmacists, drug compounding specialists and drug developers (protein based drugs).

Our IV Clear Fluid Filter
Our 0.2 µm positively charged filters protect patients from inadvertent particulate debris, microbial contaminants and their associated endotoxins for up to 96 h.

Our IV Lipid Fluid Filters
Parenteral nutrition solutions which contain lipids may require filtration through a 1.2 µm filter.

Our IV filters with Low Protein Binding Filter
Therapeutic protein products such as monoclonal antibody drugs are commonly administered parenterally by intravenous routes of administration and require low protein binding filters. Our low protein binding 0.2 µm, 1.2 µm or 5 µm membranes can be used for up to 24 hours.

Our Filters for Drug Preparation
Syringe and vent filters for pharmacists, drug compounding specialists and drug developers (protein-based drugs).
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.