Water Filters
Over 20 years ago, Pall introduced the first disposable Point-of-Use Water Filter to the market, providing a unique solution for removing waterborne pathogens delivered to the outlet from the drinking water distribution system. This unprecedented innovation brought an ease of use that revolutionized hospital hygiene and water management for healthcare facilities.

Problems
Most water management control measures cannot provide 100% peace of mind
A well-functioning water management plan often includes monitoring and control measures like flushing, secondary disinfection, and temperature regulation. However, none of these measures can guarantee that high-risk patients won't be exposed to pathogens from the water system. Some of these measures may also introduce unintended consequences into the water system1.
Read the CDC article "Reduce risk from water"
The Solution
Pall Point-of-Use Water Filters are sterilizing-grade, meaning that bacteria from the water are prevented from passing through the filter.2 Filters on all outlets in a high-risk unit are the only method that can prevent water from being a source of infection transmission. It's the simplest method in use today.
Read our blog "Water Filter Technology"
The Proof
Pall Point-of-Use Water Filters have been tested to ASTM F838-20 and have shown zero bacterial recovery,2 meaning they are sterilizing-grade according to the FDA definition.3 Their capability for safeguarding against infection has been demonstrated in numerous 3rd party studies and trials.4,5
Read our blog "About ASTM F838-20"
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014;5:258. Published 2014 Jun 2. doi:10.3389/fmicb.2014.00258
Validation Guide, Pall QPoint® Disposable Water Filter, Literature Ref.190912.95WGL
FDA, “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice”, September 2004, http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070342.pdf
Andersson K et al., “Point-of-use water filtration for providing cost effective protective care in oncology”, 35th EBMT Congress, Göteborg/Sweden, April 2009
Barna Z et al., “Tap water as a potential source of nosocomial Pseudomonas aeruginosa infections in an intensive care unit”, 19th European Congress Clin Microbiol & Infect Dis, Helsinki, Finland, Poster No° 859, May 2009
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Up to 50% of Pseudomonas aeruginosa infections can be traced back to the water system
Water outlets, such as taps and showers, are a recognized environmental reservoir for micro-organisms. Pseudomonas aeruginosa often colonizes at the point-of-use, giving it the potential to be transmitted through hand washing, bathing of infants, and aerosolization.1 This can lead to infection, antibiotic use, and increased patient stays.2
Download Infographic “Pseudomonas in water distribution systems”
The Solution
A Pall Point-of-Use Water Filter placed on the outlet can prevent transmission of Pseudomonas aeruginosa by creating a barrier between the pathogen and the outlet.
Watch webinar about pseudomonas Outbreak prevention
The Proof
Pall Point-of-Use Water Filters are sterilizing-grade according to ASTM F838-20, and has been tested with Pseudomonas aeruginosa to confirm that all of the organism is retained3. This is confirmed in field evaluations and 3rd party studies4.
Read our blog “Validation testing”
Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
Refrences
Garvey et al. (2019). Tap Out: Reducing Waterborne Pseudomonas aeruginosa Transmission in an Intensive Care Unit. J Hosp Infect, 102 (1), 75-81
Trautmann M et al. (2008). Point-of-use water filtration reduces endemic Pseudomonas aeruginosa infections on a surgical intensive care unit. Am J Infect Control, 36, 421-429
Validation Guide, Pall QPoint® Disposable Water Filter, Literature Ref.190912.95WGL
Van der Mee-Marquet N et al., “Water Microfiltration: A procedure to prevent Pseudomonas aeruginosa infection”, XVIe Congrès National de la Société Francaise d’Hygiène Hospitalière, Reims, Livre des Résumés, S137,
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Not all filters are suitable for use with high-risk patients and processes.

Pall filters use a membrane technology, instead of hollow fibers. For many hollow fiber filters, glues and resins are used to assemble the filters, providing nutrients that support bacterial and biofilm growth. These glues may also soften under warm water temperatures or with chemicals, which can lead to material leaching into the water or a breach that allows bacteria to pass through the filter. In many cases, hollow fiber filters also do not retain 100% of bacteria.1
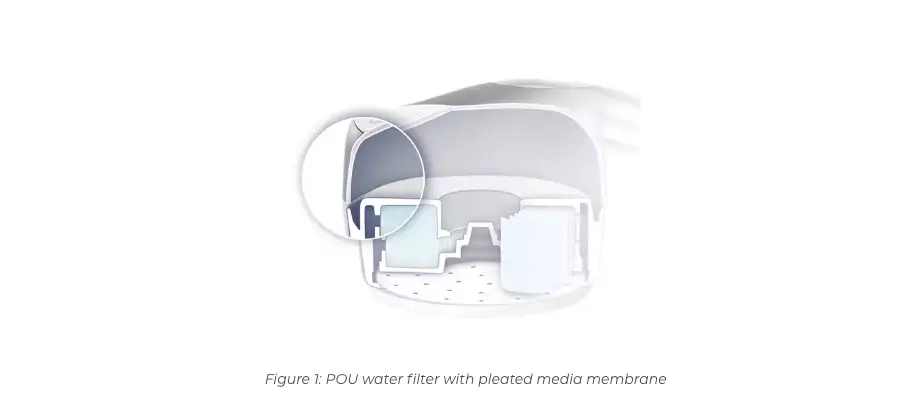
Read our blog "Water Filter Technology"
The Solution
The housing construction and sterilizing-grade membranes of Pall AquasafeTM and Pall QPoint® Point- of-Use Water Filters are assembled using mechanical methods only. No glues or resins are used in the manufacturing process of Pall Water Filters. In addition Pall Filters are 100% integrity tested upon manufacture.
The Proof
Pall's validation guide provides all information on quality, construction, and testing of the filters.2 All filters are supported by Pall's robust Quality and Scientific and Laboratory Services team should a question ever arise.
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
M. Totaro et al., Experimental comparison of point-of-use filters for drinking water ultrafiltration, Journal of Hospital Infection, 96 (2), 2017, p. 172-176, https://doi.org/10.1016/j.jhin.2016.11.017.
Validation Guide, Pall QPoint® Disposable Water Filter, Literature Ref.190912.95WGL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Instructions for Use (IFU) can be complicated and difficult to implement

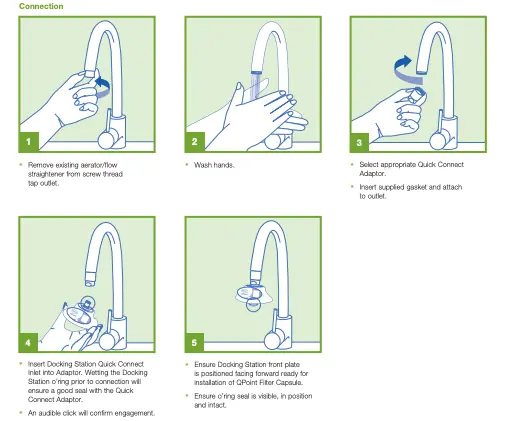
Some filters require the user to count cycles, run frequent tests on water quality, or monitor pressure drop for safe use. Liability is on the user if they fail to meet all of these instructions.
The Solution
Manual documentation to record water filter location, filter type installed, batch number, date of installation and exchange, is standard common practice. Pall Point-of-Use Water Filters are labelled to best support this practice, however manual data recording is prone to human error and can be time consuming. Click below to learn how the Pall XChangr supports simplified documentation.
The Proof
Filters can be used at pressures up to 75 psi and within a wide temperature range. They can even be used for a period of time with chemical and heat shock treatments. Filter life is clearly stated. No additional cycle counting or water quality testing is necessary for safe use.
Read the Blog "What is a POU water filter".
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
Technical Text, Point-of-Use Disposable Water Filters, Literature Ref. 190930.2WGL
Data on file, please contact Pall for additional information.
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Compatibility with Systemic Treatments
Systemic disinfectants are frequently used as a supporting control measure in water distribution systems.1 Point-of-Use Water Filters should therefore be compatible with and their performance not affected by commonly used systemic disinfectants, like chlorine, monochloramine, and chlorine dioxide.
The Solution
Pall Point-of-Use Water Filters are compatible with a wide range of chemicals commonly used for systemic disinfection.
Download infographic “Systemic disinfection solution”
Read our blog “Point-of-use water filter technology”
The Proof
Our Filters can be used with the following treatments
1. Active chlorine:
Continuous treatment: < 4 ppm at ambient temperature
Shock treatment: 100 ppm active chlorine for 1 hour at ambient temperature
2. Monochloramine:
Continuous shock treatment: 2.2 ppm over 9 hours
3. Peracetic acid:
Shock treatment: 1000 ppm at 60 °C for 2 hours at ambient temperature
4. pH >12:
Shock treatment: for up 1 h at ambient temperature
5. Chlorine dioxide:
Continuous treatment: < 1 ppm at ambient temperature- shock treatment:100 Mg/L for 12 hours at ambient temperature
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
Data on file, please contact Pall for additional information.
Healthcare Water Management Team Bulletin, Synergistic Water Hygiene Control Measures: A Multi-Barrier Approach, Literature Ref.180604.1WGL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Chemical Compatibility with Surface Disinfectants

A number of disinfectants are used in patient care areas to protect surfaces. Point-of-Use Water Filters should be compatibile with these disinfectants and resist cracking or leaking when cleaned.
The Solution
Pall-AquasafeTM and Pall QPoint® are compatible with commonly used surface disinfectants such as active chlorine, iso-propyl alcohol, quaternary ammonium compounds, benzyl ammonium chlorides, and non-ionic surfactants.
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
Data on file, please contact Pall for additional information
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Endoscopes haven't been cleaned properly
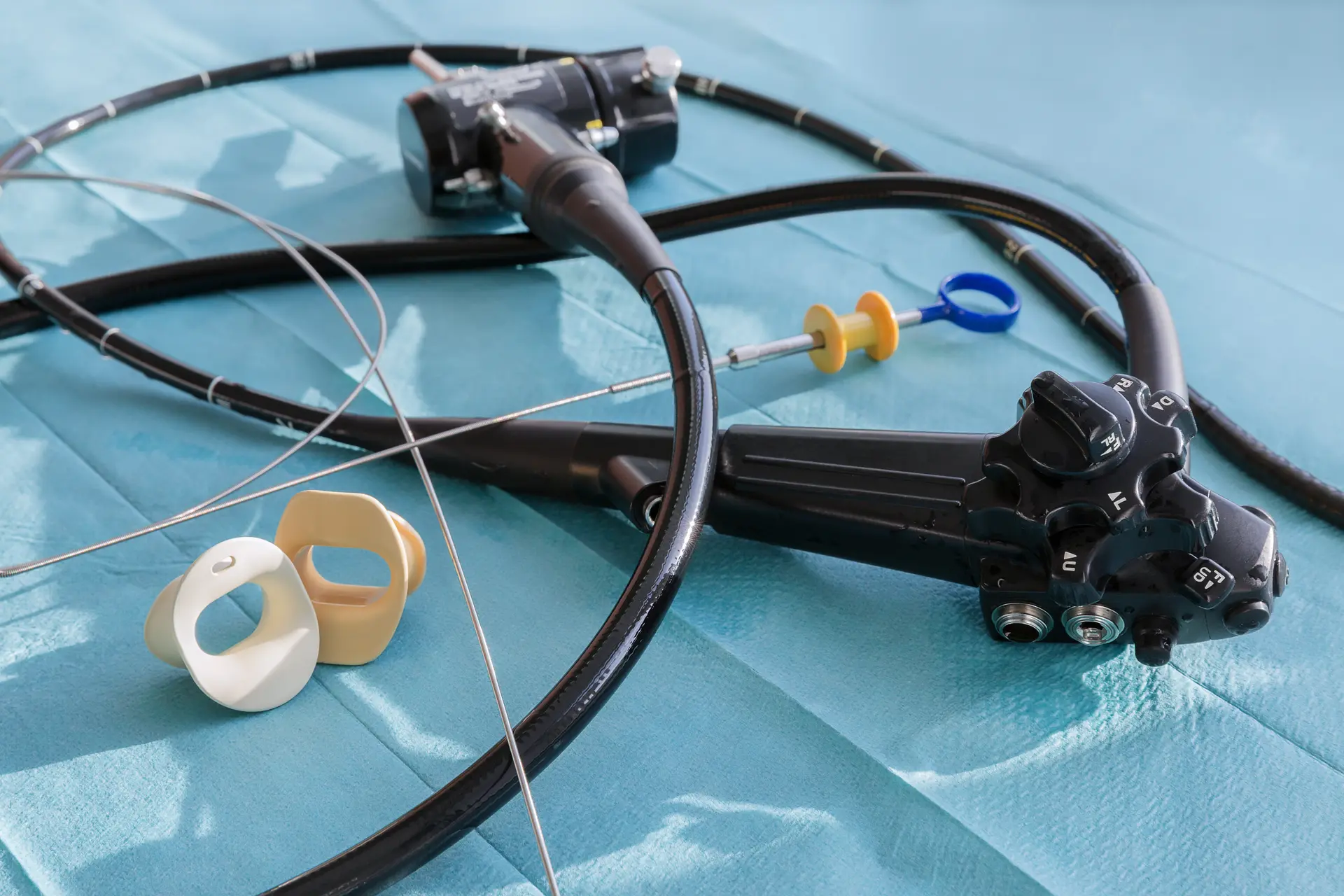
The standards for cleaning of scopes are increasingly strict because scopes can be difficult to reprocess properly. The quality of the water plays a big part on how well your Automated Endoscope Reprocessor is able to clean and often requires additional filtration outside of the machine to reduce debris, bacteria, or endotoxin.1
The Solution
Pall has an extensive portfolio of filtration to protect the filters inside the AER. This portfolio ranges from inexpensive prefilters for debris to innovative solutions to reduce bacteria or endotoxin in the water.2
Download infographic “Water Treatment for Endoscope Reprocessing”
The Proof
Download “Endoscope cleaning solution”
Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
World Gastroenterology Organisation, "Endoscope disinfection update: a guide to resource-sensitive reprocessing", April 2019, https://www.worldgastroenterology.org/guidelines/endoscope-disinfection/endoscope-disinfection-english
Water Filtration Solutions for Endoscope Reprocessing, Lit. Ref. 210601.1WGL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Automated Endoscope Reprocessor (AER) filters have clogged prematurely

If one of these filters clogs, it typically means that the cycle must restart after the filter has been replaced. Replacing these filters can be expensive because these filters remove all types of contaminants from the water. However, depending on water quality, those filters might not be enough and additional filtration might be required outside of the machine.1
The Solution
Pall has an extensive portfolio of filtration to improve the water quality and protect the filters inside the AER. This portfolio ranges from inexpensive prefilters for debris to innovative solutions to reduce bacteria or endotoxin in the water.
Download infographic “AER Clogging”
The Proof
Download brochure “Water filtration solution for endoscope reprocessing”
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
Water Filtration Solutions for Endoscope Reprocessing, Lit. Ref. 210601.1WGL
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.
Rinse water for endoscopes should be sterile

Water used to rinse or flush endoscopes should be of high purity so as not to introduce pathogens1. Sterile or sterilizing-grade quality water is considered suitable, but sterile water can be expensive and unwieldy.
Download infographic “Endoscope cleaning solution”
The Solution
Pall Point-of-Use Water Filters provide sterilizing-grade water2 according to ASTM F838-20 and the FDA definition3. For this reason, it is considered suitable for use in rinsing medical instruments.
Read our blog “The importanceof fda 510k clearance for medical devices”Read Blog Post
The Proof
Pall Point-of-Use Water Filters have been tested to ASTM F838-20 and have shown zero bacterial recovery2, meaning they are sterilizing-grade according to the FDA definition3.
Our Products
Since introducing the first disposable point-of-use (POU) water filter to the market over 20 years ago, we’ve worked to help improve hospital hygiene and water management strategies within diverse facility settings.

Our POU Water Filters for 31-Day Use
Our Aquasafe™ 0.2 µm sterilizing-grade filters are great for outbreak response, high-contamination environments, or anywhere where the utmost risk reduction is critical.

Our POU Water Filters for Long-Term Use with High Risk Patients
Our QPoint® filters are 0.2µm sterilizing-grade filters, engineered to minimize waterborne pathogen risk at taps and showers over a longer life.

Our Prefilters
Our prefiltration portfolio prepares the water for use in an entire building or for critical processes by removing debris and other contaminants.

Our Process Filters
Our portfolio of process filters help protect from endotoxin and microorganisms in areas like dialysis, sterile processing, and endoscope reprocessing.
References
1. Willis C. Bacteria-free endoscopy rinse water -- a realistic aim?. Epidemiol Infect. 2006;134(2):279-284. doi:10.1017/S0950268805005066
2. Validation Guide, Pall QPoint® Disposable Water Filter, Literature Ref.190912.95WGL
3. FDA, “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice”, September 2004, http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070342.pdf
The products advertised within this website may not have been licensed in accordance with local regulatory laws. Please check with the local Pall office for availability.